The Theme of Unity and Diversity in Biomolecules and the Chemistry of Life
As you think about biological molecules, it’s easy to see how the theme of unity and diversity applies. For starters, all biological molecules use a relatively small number of building blocks—our favorite word, monomers—to make a diverse array of larger polymers. You might even call them biopolymers. Within each class of biomolecules, carbohydrates, lipids, proteins, and nucleic acids, there is unity in the fact that the same monomers are used again and again, and diversity results from putting the monomers together in different ways.
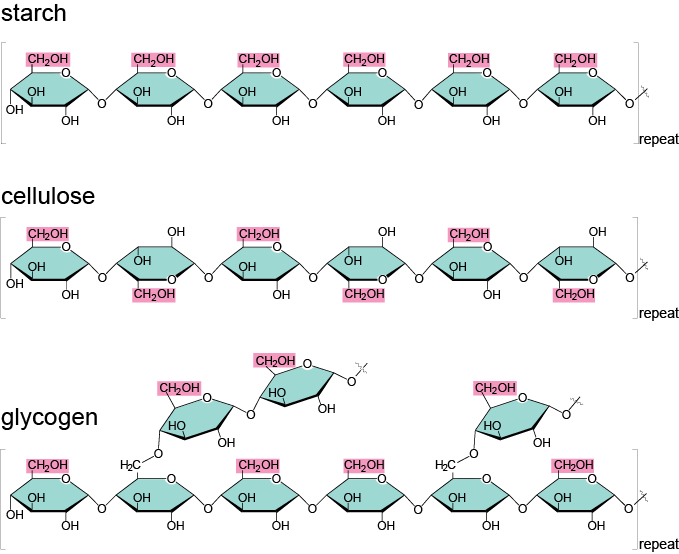
Carbohydrates exemplify this theme, so let’s take a closer look at the wide variety of polysaccharides that exist and what gives rise to this diversity. We mentioned earlier, in the In Depth section, that polysaccharides are used for energy storage. The polysaccharide of choice for plants is starch. Starch is basically a whole bunch of glucose monomers stuck together. When plants need energy, they use enzymes to break the bonds between glucose molecules one by one. Plants will only release as much glucose as they need at the moment, which is kind of nifty when you think about it. Plants are practical like that. Most animals also have enzymes that allow them to break plant starch into sugars, which is the reason that starchy foods like grains and potatoes are such a good source of energy.
It turns out that there are different kinds of starches, and these different types arise partly as the result of chemical bonds. Remember that monosaccharides form rings before they bond together; depending on which carbon in the first monomer binds with the second monomer, you can get pretty different structures.
Now, let us be the Friz to your Arnold. Seatbelts everyone! Glucose is a 6-carbon ring. Therefore, if the fourth carbon of one glucose monomer binds with the first carbon of a second glucose monomer, and this happens lots and lots of times so that lots and lots of bonds form, we will have a chain with no branches. (In fact, this starch is called amylose.) However, if some glucose molecules deviate from this bonding pattern, we can get a branched form of starch. Different starches are united by the fact that they have the exact same building blocks, and diversity arises when those building blocks are assembled in different ways.
In animals, the storage polysaccharide is called glycogen. Glycogen has lots of branches and is stored in liver and muscle cells. We animals are like plants in that we can release glucose from the polysaccharide as needed, but glycogen stores do not last very long: less than a day in humans, and far less in some other animals. Shucks.
So far, we’ve seen how carbohydrates can be used immediately or stored for later use. On top of the majorly important task of providing energy, some carbohydrates play an important role in providing structural support. In plants, the main structural polysaccharide is cellulose. Cellulose constitutes most of the cell walls of plants (animal cells don't have cell walls). Ecologists, or those guys who study how organisms relate to their physical surroundings, that want to impress their friends like to say that over a trillion tons of cellulose are produced on Earth each year – that is more than the weight of 200 billion elephants. That’s a lot of elephants.
Cellulose, like starch and glycogen, is made from glucose monomers, but they bond together in a different way. In the storage molecules starch and glycogen, all the glucose molecules are right-side-up, and animals have enzymes that can deal with these kinds of bonds. In cellulose, however, every other glucose monomer is upside down, creating a different sort of bond. Animal enzymes are perplexed by this and cannot figure out how to break these bonds. Some animals, like cows, have microbes in their digestive tracts that are able to break these bonds, and they are some of a very few that can get a little energy from cellulose. For everyone who isn’t lucky enough to have a little clan of helpful, cellulose-digesting microbes, including us humans, cellulose passes through our systems undigested. This cellulose is famously known as fiber. Metamucil, anyone?
Picture time! Here are the chemical structures for these sugars. Remember, no central atom means it is carbon, and any needed hydrogen atoms are "assumed."
Chitin is another structural polysaccharide, but this one is found in fungi (mushrooms and whatnot) and some animals. It’s made of glucose like the other polysaccharides but has some nitrogen thrown in the mix for good measure. Chitin comprises the hard exoskeleton of arthropods, like insects and crustaceans, and strengthens the cell walls of fungi. Since fungi and animals share a more recent common ancestor than either one shares with plants, it makes some evolutionary sense that animals and fungi share this important structural polysaccharide. Yes, you read that correctly. A mushroom is more closely related to you than it is to any plant. Hey, everyone’s extended family has a few nuts. Er, mushrooms.
The take-home point here is that there is an array of storage and structural polysaccharides, but they are unified by a common monosaccharide building block: glucose.
Unity and diversity apply to other biomolecules, too. Think about DNA: the same four nucleotides combined in different ways are the basis for all the diverse life forms on Earth! Similarly, just 22 amino acids serve as the common building blocks for a plethora of protein structures. There is also unity in the way that monomers tend to be bound together. In all the biological molecules we looked at, monomers were assembled by dehydration synthesis. The fact that so much of life’s diversity is attributable to so few building blocks and processes is truly amazing. It would be like building New Orleans, with all its richness, color, and vigor, out of 3D puzzle pieces. Let’s be glad that Mother Nature is a more skilled architect than we are!