Enzymes in Detail
Enzymes are like the fast-forward button on your DVR remote. They buzz about 24/7 and involve themselves in chemical reactions, yet they always have these two properties:
We can study enzymes in the context of activation energy. Many biochemical reactions need a little input of energy to jump-start a thermodynamically favorable reaction. The activation energy is the amount of energy needed for the reaction to go forward and get over its activation barrier.
ATP (adenosine triphosphate), the cell's energy molecule, needs a little help to get over its activation barrier. Otherwise, ATP might donate its terminal (read: end) phosphate group prematurely, resulting in an untimely release of energy. That would be bad—very bad. The cell makes sure that a reaction occurs when and where it wants by controlling the availability and abundance of enzymes.
The need to reach the activation energy can be compared to when a roller coaster needs to be "pulled" up the track and to the top of the hill before it can go rolling down at exhilarating speeds. Until the coaster makes it over the hump, it won't be able to proceed down the other side. It's helpful to look at chemical reactions using an energy diagram (see below). Enzymes lower the activation energy of desired reactions and kick-start them to get those reactions rolling.

Enzymes lower the activation energy of a reaction by binding one of the reactants, called a substrate, and holding it in a way that lowers the activation energy. Say, for instance, that the reaction is the event of hitting a baseball. Your bat needs to come into contact with the ball. One option is for Brian Wilson (The Beard) to show up dressed to the spandexed nines and pitch the ball to you. Good luck hitting the ball when you have such a glorious, uh, beard to look at.
Alternatively, you could play tee-ball. Yes, tee-ball. Not as cool as the previous option, for sure, but now you're much more likely to have bat meet ball. Having an enzyme around is a lot like putting a baseball on a tee to increase the chances of collision between the ball and the bat because it holds the ball in place. Likewise, an enzyme holds its substrate in such a way that the reaction is much more likely to occur.
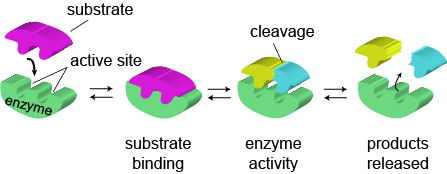
We mentioned earlier that enzymes are often present in small, controlled amounts. Amazingly, it's estimated that a typical enzyme will catalyze the reaction of about a thousand substrate molecules every second.1
How efficient an enzyme is at catalyzing a reaction is dependent on the reaction conditions and on how good the enzyme is at finding its substrate. Molecules in the cell are constantly in motion, wandering around the cell in a process called diffusion. In diffusion, molecules move from areas of higher concentration to lower concentration.
You can imagine, though, that the chance that any enzyme will meet its substrate is dependent on how much substrate is in the cell. In this case, the substrate is the limiting factor of the reaction rate, slowing and eventually preventing any further reactions from occurring in its absence. If there is little substrate, the enzyme is less likely to find the substrate and catalyze the reaction.
Alternatively, if the substrate concentration increases and reaches a high amount, the reaction rate becomes dependent on the limiting characteristics of the enzyme. An enzyme is considered to be working at its maximal rate under this condition, where the amount of substrate exceeds the enzyme capacity. In this way, the cell directly controls the rate of reaction by controlling the amount of enzyme available to the substrate.
Enzymes are usually extremely specific, meaning that one enzyme only catalyzes one type of biochemical reaction. How is the specificity of an enzyme determined? Their pickiness comes from the enzyme's active site, a unique binding site that only a particular substrate will recognize and be able to fit inside. The active site isn't changed after an enzyme catalyzes a reaction, so a new substrate can still fit in the site when the old substrate has gone away.
There are specific enzymes for each biological reaction. Most biological reactions are also connected, meaning that the product from one enzyme-catalyzed reaction (say, enzyme A) is often used as a reactant in another reaction catalyzed by a different enzyme (say, enzyme B). The result is a complicated network of biochemical reactions.

Because enzymes are proteins, they can be a bit fragile. Like all proteins, an enzyme is only as good as its structure. Things like temperature, pH, or salt content can take a properly folded enzyme and turn it into garbage. A protein that isn't neatly folded can't do its job. This is called denaturation, and it spells disaster for any enzyme.
At high temperatures, for example, the shape of an enzyme can change, and if that happens, it's likely that the active site will look different. The substrate won't fit into the new site, meaning the enzyme is pretty much useless.
Changes to pH can also wreak havoc on protein function. For instance, enzymes in the stomach are uniquely suited to function at very low pH. Take those enzymes to another part of the body where the pH is neutral, and they’ll fall apart faster than a preteen at a Bieber concert. Likewise, a blood enzyme that’s accustom to the slightly basic pH of 7.4 isn’t going to make it in the pH 2 environment of the stomach.
Own a pair of stonewashed jeans? Think again! Jean manufacturers now use enzymes instead of stones to give jeans that stonewashed look by degrading the denim fabric. This method actually damages the jeans less than the stone method.
- They don't change the thermodynamic properties of the reaction.
- They aren't consumed or modified in the reaction.
We can study enzymes in the context of activation energy. Many biochemical reactions need a little input of energy to jump-start a thermodynamically favorable reaction. The activation energy is the amount of energy needed for the reaction to go forward and get over its activation barrier.
ATP (adenosine triphosphate), the cell's energy molecule, needs a little help to get over its activation barrier. Otherwise, ATP might donate its terminal (read: end) phosphate group prematurely, resulting in an untimely release of energy. That would be bad—very bad. The cell makes sure that a reaction occurs when and where it wants by controlling the availability and abundance of enzymes.
The need to reach the activation energy can be compared to when a roller coaster needs to be "pulled" up the track and to the top of the hill before it can go rolling down at exhilarating speeds. Until the coaster makes it over the hump, it won't be able to proceed down the other side. It's helpful to look at chemical reactions using an energy diagram (see below). Enzymes lower the activation energy of desired reactions and kick-start them to get those reactions rolling.

Enzymes lower the activation energy of a reaction by binding one of the reactants, called a substrate, and holding it in a way that lowers the activation energy. Say, for instance, that the reaction is the event of hitting a baseball. Your bat needs to come into contact with the ball. One option is for Brian Wilson (The Beard) to show up dressed to the spandexed nines and pitch the ball to you. Good luck hitting the ball when you have such a glorious, uh, beard to look at.
Alternatively, you could play tee-ball. Yes, tee-ball. Not as cool as the previous option, for sure, but now you're much more likely to have bat meet ball. Having an enzyme around is a lot like putting a baseball on a tee to increase the chances of collision between the ball and the bat because it holds the ball in place. Likewise, an enzyme holds its substrate in such a way that the reaction is much more likely to occur.

We mentioned earlier that enzymes are often present in small, controlled amounts. Amazingly, it's estimated that a typical enzyme will catalyze the reaction of about a thousand substrate molecules every second.1
How efficient an enzyme is at catalyzing a reaction is dependent on the reaction conditions and on how good the enzyme is at finding its substrate. Molecules in the cell are constantly in motion, wandering around the cell in a process called diffusion. In diffusion, molecules move from areas of higher concentration to lower concentration.
You can imagine, though, that the chance that any enzyme will meet its substrate is dependent on how much substrate is in the cell. In this case, the substrate is the limiting factor of the reaction rate, slowing and eventually preventing any further reactions from occurring in its absence. If there is little substrate, the enzyme is less likely to find the substrate and catalyze the reaction.
Alternatively, if the substrate concentration increases and reaches a high amount, the reaction rate becomes dependent on the limiting characteristics of the enzyme. An enzyme is considered to be working at its maximal rate under this condition, where the amount of substrate exceeds the enzyme capacity. In this way, the cell directly controls the rate of reaction by controlling the amount of enzyme available to the substrate.
Enzymes are usually extremely specific, meaning that one enzyme only catalyzes one type of biochemical reaction. How is the specificity of an enzyme determined? Their pickiness comes from the enzyme's active site, a unique binding site that only a particular substrate will recognize and be able to fit inside. The active site isn't changed after an enzyme catalyzes a reaction, so a new substrate can still fit in the site when the old substrate has gone away.
There are specific enzymes for each biological reaction. Most biological reactions are also connected, meaning that the product from one enzyme-catalyzed reaction (say, enzyme A) is often used as a reactant in another reaction catalyzed by a different enzyme (say, enzyme B). The result is a complicated network of biochemical reactions.

Because enzymes are proteins, they can be a bit fragile. Like all proteins, an enzyme is only as good as its structure. Things like temperature, pH, or salt content can take a properly folded enzyme and turn it into garbage. A protein that isn't neatly folded can't do its job. This is called denaturation, and it spells disaster for any enzyme.
At high temperatures, for example, the shape of an enzyme can change, and if that happens, it's likely that the active site will look different. The substrate won't fit into the new site, meaning the enzyme is pretty much useless.
Changes to pH can also wreak havoc on protein function. For instance, enzymes in the stomach are uniquely suited to function at very low pH. Take those enzymes to another part of the body where the pH is neutral, and they’ll fall apart faster than a preteen at a Bieber concert. Likewise, a blood enzyme that’s accustom to the slightly basic pH of 7.4 isn’t going to make it in the pH 2 environment of the stomach.
The Diversity of Enzymes
The different types of enzymes can be divided into groups based on the types of reactions they catalyze.| Type of Enzyme | Enzyme Function |
|---|---|
| Nuclease | Cleaves the bond connecting two nucleic acids. |
| Protease | Catalyzes the disruption of the bonds that connect amino acids in a protein. |
| Polymerase | Catalyzes the production of biological polymers, such as RNA and DNA. |
| Kinase | Adds a phosphate to one biological molecule through a process called phosphorylation (very creative). Kinases are important signaling molecules. |
| ATPase | Catalyzes the conversion of ATP into ADP, which releases energy to drive cellular processes. |
| Phosphatase | Catalyzes the opposite reaction of a kinase by removing a phosphate group |
Brain snack
Ever wonder why apple slices turn brown? Apples brown because of a family of enzymes called PPO (polyphenol oxidase, if you must know). These enzymes catalyze a reaction between the oxygen in the air and the iron-containing compounds in apples. The result? Enzymatic browning.Own a pair of stonewashed jeans? Think again! Jean manufacturers now use enzymes instead of stones to give jeans that stonewashed look by degrading the denim fabric. This method actually damages the jeans less than the stone method.