The United States of Matter
As we've already said, matter is anything that has mass and occupies space. We're not just talking about the moon and stars, either. We mean the sort of space that takes up volume. Matter can exist in four states: solid, liquid, gas, or plasma.
Particles of matter behave differently depending on whether they're in the solid, liquid, or gas phase. We'll leave plasma out of the story for now, but you can bet your bottom dollar we'll cover it later. When we refer to a particle we just mean a small unit of matter and it is a word we use for convenience. The particles in neon gas, for example, are neon atoms, while the particles in laughing gas are N2O molecules.
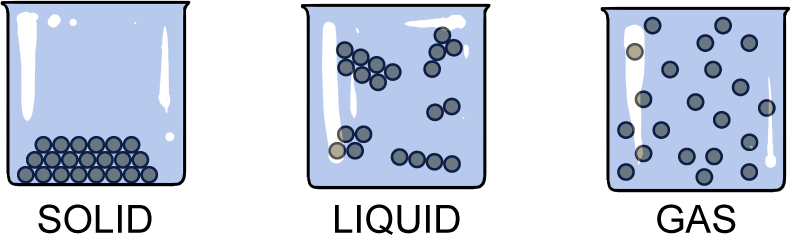
Check out the nifty illustration below. The particles in the different states are characteristically organized (or disorganized in some states).
How might we go about measuring the volume of an ice cube? The easiest way is to use the volume displacement method. This method is used to find the volume of irregularly shaped objects by measuring the level of water before and after immersing a solid into a graduated cylinder of water. Check out this video demonstration here.
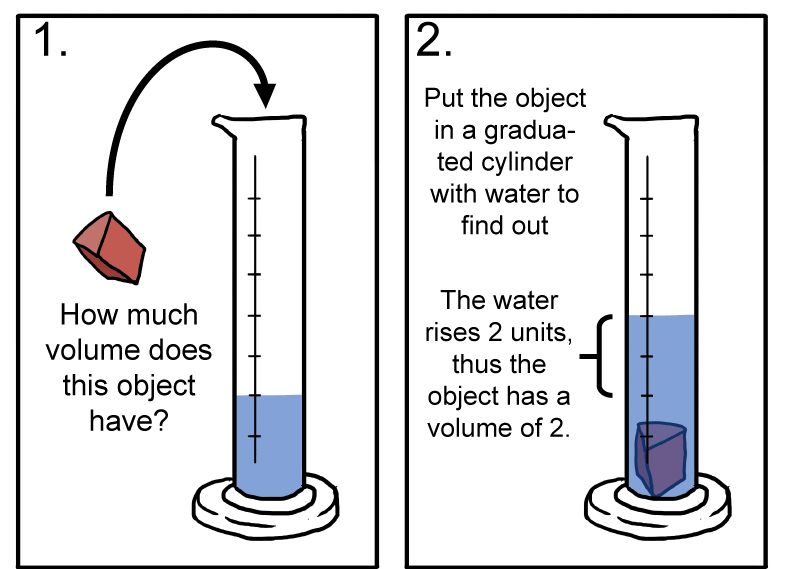
The volume displacement method.
Okay! Okay! We'll get back to solids already!
At the microscopic level, the particles that make up the solid are very close together and aren't moving around very much. This is because in many solids, the particles are pulled into a rigid, organized structure of repeating patterns called a crystal lattice. The particles in the crystal lattice are still moving but barely. Picture an awkward dance party where everyone is solo and stiffly trying to dance in place. Sounds like middle school.
One common term used to describe solids is malleability. This word can be used to describe a physical property of all matter, but it is usually most relevant in the case of solids. Malleability is the ability of matter to bend or be hammered into other shapes without breaking. Examples of highly malleable metals are gold, iron, and aluminum. We've all experienced malleability first hand when using aluminum foil. Think of how easy it is to mold aluminum into the shape we want!

Aluminum foil does whatever you tell it to do. It's malleable, and we like it that way. (Image from here.)
You'll learn more than you ever wanted to learn about crystal lattices (and the microscopic properties of liquids and gases) later on. Let's continue with our introduction.
Even though the particles in liquids are farther apart, there are a few particles that may still be near each other, clumped together in small groups. This is all thanks to the attractive forces between particles. The attractive forces among the liquid particles aren't as strong as they are in solids. This explains why liquids do not have a definite shape. The forces are however strong enough to keep the substance confined in one large mass instead of going all over the place.
One term often associated with liquids is the always fun-to-say viscosity (visk-os-city). We've all heard the term before, but what does it actually mean? This question is often best answered by an example.
Imagine a Styrofoam cup with a hole in the bottom. If we pour some honey into the cup, we would find that the cup drains very slowly. That is because honey's viscosity is large compared to, say, water's viscosity. If we fill the same cup with water instead, the cup will drain much more quickly because water's viscosity is much lower than that of honey.

Me want honeycomb—it's so viscous. (Image from here.)
Viscosity is a measure of a fluid's resistance to flow. It describes the internal friction of a moving fluid. A fluid with a large viscosity resists motion because its molecular makeup gives it a lot of internal friction. A fluid with a low viscosity flows easily because its molecular makeup results in very little friction when it's in motion.

Liquids have attractive forces but they are weaker than in solids. (Image from here.)
Because of the distance between the particles and the independent motion of each of them, the gas expands to fill the area that contains it. This also explains why gases have no definite shape. Two words: balloon animals.

Gases taking the shape of a turkey. (Image from here.)
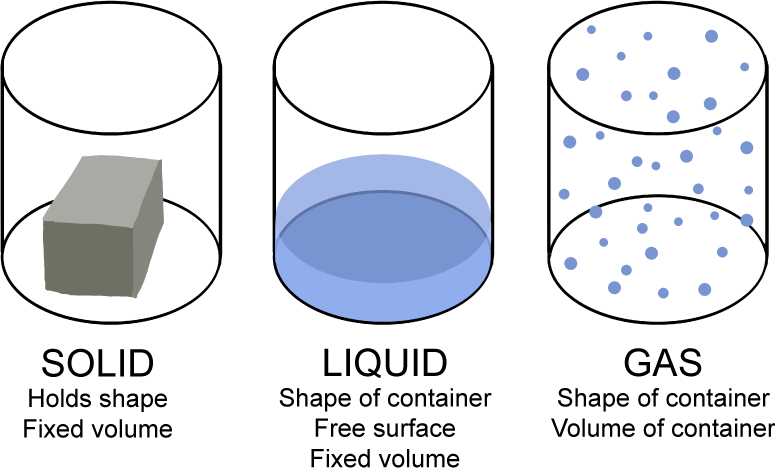
Let's review the basics of solids, liquids, and gases in one simple little illustration.
Just to make sure we're all on the same page let's compare neon gas to neon plasma. In neon gas, the electrons are all bound to the nucleus. In neon plasma, the electrons are free to move around the system. Plasma is kind of like an out-of-control super-hyper gas.

Whoa. Plasma. (Image from here.)
If we were to go searching for naturally occurring plasma, we wouldn't find it easily because it doesn't regularly occur on Earth. It takes a special environment for plasma to form and keep going. While natural plasmas aren't too common on Earth, man-made plasmas are everywhere in the form of fluorescent light bulbs and neon signs.
Particles of matter behave differently depending on whether they're in the solid, liquid, or gas phase. We'll leave plasma out of the story for now, but you can bet your bottom dollar we'll cover it later. When we refer to a particle we just mean a small unit of matter and it is a word we use for convenience. The particles in neon gas, for example, are neon atoms, while the particles in laughing gas are N2O molecules.
Check out the nifty illustration below. The particles in the different states are characteristically organized (or disorganized in some states).

Solids
Pick up your favorite solid. The possibilities are endless. What do you see at the macroscopic level? This is the size level where we can directly observe with our senses. No microscope required. Each solid has a definite shape and occupies a definite volume. Think of an ice cube in a glass. It's a solid. We can easily weigh the ice cube and measure its volume.How might we go about measuring the volume of an ice cube? The easiest way is to use the volume displacement method. This method is used to find the volume of irregularly shaped objects by measuring the level of water before and after immersing a solid into a graduated cylinder of water. Check out this video demonstration here.

The volume displacement method.
Okay! Okay! We'll get back to solids already!
At the microscopic level, the particles that make up the solid are very close together and aren't moving around very much. This is because in many solids, the particles are pulled into a rigid, organized structure of repeating patterns called a crystal lattice. The particles in the crystal lattice are still moving but barely. Picture an awkward dance party where everyone is solo and stiffly trying to dance in place. Sounds like middle school.
One common term used to describe solids is malleability. This word can be used to describe a physical property of all matter, but it is usually most relevant in the case of solids. Malleability is the ability of matter to bend or be hammered into other shapes without breaking. Examples of highly malleable metals are gold, iron, and aluminum. We've all experienced malleability first hand when using aluminum foil. Think of how easy it is to mold aluminum into the shape we want!

Aluminum foil does whatever you tell it to do. It's malleable, and we like it that way. (Image from here.)
You'll learn more than you ever wanted to learn about crystal lattices (and the microscopic properties of liquids and gases) later on. Let's continue with our introduction.
Liquids
Unlike solids, liquids do not have a definite shape, kind of like Slimer from Ghostbusters. They do, however, have a definite volume like solids. The particles in liquids are much farther apart than the particles in solids. They're also moving around much more. These guys are a little more fun at a dance party. They're still dancing solo but now they're moving around the room to the beat of will.i.am.Even though the particles in liquids are farther apart, there are a few particles that may still be near each other, clumped together in small groups. This is all thanks to the attractive forces between particles. The attractive forces among the liquid particles aren't as strong as they are in solids. This explains why liquids do not have a definite shape. The forces are however strong enough to keep the substance confined in one large mass instead of going all over the place.
One term often associated with liquids is the always fun-to-say viscosity (visk-os-city). We've all heard the term before, but what does it actually mean? This question is often best answered by an example.
Imagine a Styrofoam cup with a hole in the bottom. If we pour some honey into the cup, we would find that the cup drains very slowly. That is because honey's viscosity is large compared to, say, water's viscosity. If we fill the same cup with water instead, the cup will drain much more quickly because water's viscosity is much lower than that of honey.

Me want honeycomb—it's so viscous. (Image from here.)
Viscosity is a measure of a fluid's resistance to flow. It describes the internal friction of a moving fluid. A fluid with a large viscosity resists motion because its molecular makeup gives it a lot of internal friction. A fluid with a low viscosity flows easily because its molecular makeup results in very little friction when it's in motion.

Liquids have attractive forces but they are weaker than in solids. (Image from here.)
Gases
A gas has no definite shape and no definite volume. In a gas, the particles are much farther apart than they are in solids or liquids. The particles are also moving more independent of each other. Now we're talking about a wild dance party. We might even see a few crazy people bouncing off the walls.Because of the distance between the particles and the independent motion of each of them, the gas expands to fill the area that contains it. This also explains why gases have no definite shape. Two words: balloon animals.

Gases taking the shape of a turkey. (Image from here.)
Let's review the basics of solids, liquids, and gases in one simple little illustration.

Plasma
Now things are about to get weird. The fourth state of matter is plasma. This wacky state-of-matter is similar to the gas phase, but the particles of plasma are free electrons and ions of an element. In other words, plasma is made up of groups of positively charged particles (the ions of the element) and negatively charged particles (the free electrons).Just to make sure we're all on the same page let's compare neon gas to neon plasma. In neon gas, the electrons are all bound to the nucleus. In neon plasma, the electrons are free to move around the system. Plasma is kind of like an out-of-control super-hyper gas.

Whoa. Plasma. (Image from here.)
If we were to go searching for naturally occurring plasma, we wouldn't find it easily because it doesn't regularly occur on Earth. It takes a special environment for plasma to form and keep going. While natural plasmas aren't too common on Earth, man-made plasmas are everywhere in the form of fluorescent light bulbs and neon signs.