ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Laws of Thermodynamics Videos
Play All
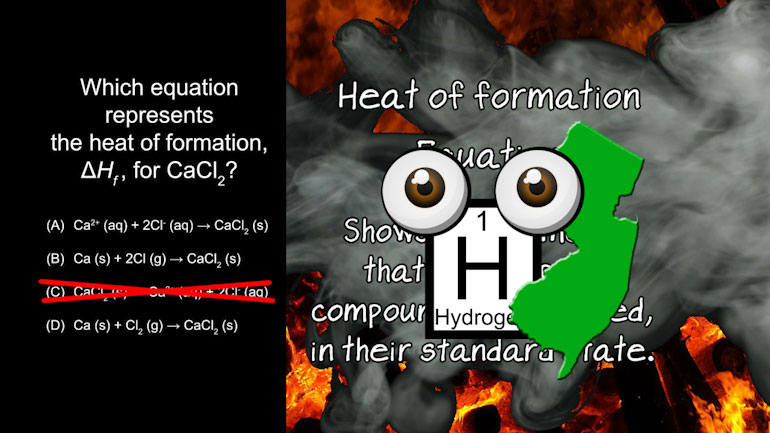
AP® Chemistry: Laws of Thermodynamics Drill 1, Problem 1. Which equation represents the heat of formation for Calcium Chloride?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?
AP Chemistry 3.1 Laws of Thermodynamics. What is the change in enthalpy of this reaction?
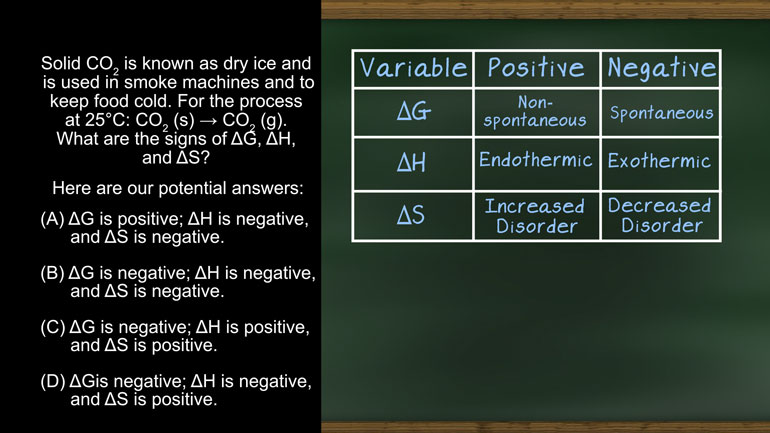
AP Chemistry 2.4 Laws of Thermodynamics. What are the signs of ΔG, ΔH, and ΔS?
AP Chemistry 2.2 Laws of Thermodynamics. What is the ΔG and the spontaneity of the reacton?
AP Chemistry 1.5 Laws of Thermodynamics. What is the heat of combustion of propene C3H6?
AP Chemistry 1.4 Laws of Thermodynamics. Which of the following statements is true?
AP Chem 1.3 Laws of Thermodynamics. How much energy would be required to change the temperature of 200 g of aluminum as described in the video?
AP Chemistry 1.2 Laws of Thermodynamics. What is the standard enthalpy for the following reaction?
This is a question about Gibbs free energy. You'd better act fast though. Otherwise, you may have to pay the normal sticker price of $99.99.
AP Chemistry 3.3 Laws of Thermodynamics. How much energy would be required to completely melt the ice?
AP Chemistry 3.4 Laws of Thermodynamics. What is the specific heat of the metal?
AP Chemistry 2.5 Laws of Thermodynamics. Which of the following is true for a reaction at equilibrium?
AP Chemistry 3.5 Laws of Thermodynamics. Which of the following is true regarding state functions?