Particle Physics
The difference between nuclear and atomic physics and particle physics is that nuclear and atomic physics concern matter in atoms and nuclei. Particle physics deals primarily with the interactions of particles outside of an atom or nucleus. Well, for the most part. It includes the addition of particles to nuclei so the boundaries between particle and nuclear physics blur. We'll just go with it.
It certainly seemed bizarre to us. So bizarre that we had to send in one of our Shmoop particle detectives to investigate this large mass difference.
Something with a larger mass might be heavier. We say might because it also depends on density. Protons and neutrons, as it turns out, are definitely larger than an electron. In fact, an electron doesn't even have a definite size, since they're described by a wave function and quantum properties just as much as by particle ones.
As far as we know and, more importantly, as far as scientists know, we can't break an electron into any smaller pieces. The electron is thus an elementary particle. It's also a lepton, subject to the weak nuclear force, but not the strong one. All leptons happen to be elementary particles. The leptons are: electrons, muons, taus, positrons,which are the opposite of electrons, and neutrinos.
Nucleons, on the other hand, aren't elementary particles, they are composite particles. It's a twist, we know, and it may come as a surprise, but neutrons and protons are made up of other particles: quarks.
Like electrons, quarks are elementary particles too, and there are several kinds of quarks. Quarks are not leptons, though, they are hadrons, which means they are subject to the strong nuclear force, but not the weak one. A baryon is a particle composed of three quarks. The two most famous baryons are—we knew it—protons and neutrons.
Let's back up for a moment and talk about forces. Some leptons and hadrons obey the electromagnetic force, but not all of them. To feel the electromagnetic force, a particle must have a charge. Neutrinos (a lepton) are neutral; neutrons (a hadron) have no charge either; but the electromagnetic force does not bother with neutrinos and neutrons. However, the weak and strong nuclear forces certainly bother with them. That tells us that the strong and weak forces are independent of the electromagnetic force. Now, let's go back to talking about quarks.
We just learned that both neutrons and protons are baryons with three quarks, but protons, unlike the neutral neutrons, have a positive charge. How can this be? How can some baryons be charged while others aren't? That's because the quarks are different for protons and neutrons.
Quarks can take on six types of flavors, and yes, they're actually called flavors. We don't have to worry about most of them. The only flavors we care about are up quarks and quarks. Up quarks have a charge of . Down quarks have a charge of
. Down quarks have a charge of 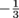 . Physicist discovered this through experimentation with particle accelerators in the 90's.
. Physicist discovered this through experimentation with particle accelerators in the 90's.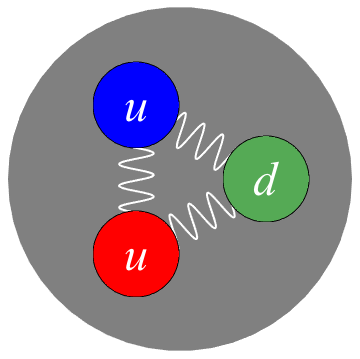 Pictures really are worth a thousand words. Put a
Pictures really are worth a thousand words. Put a 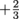 in all the up quarks, and a
in all the up quarks, and a  in the down ones, and lo and behold, protons have a charge of + 1 and neutrons have no charge.
in the down ones, and lo and behold, protons have a charge of + 1 and neutrons have no charge.  .
.
When an unstable radioactive isotope undergoes alpha decay, it loses 2 neutrons and protons and decays into something else. Let's take a look at the following reaction:
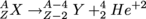
where X is the unstable radioactive parent isotope, Y is the product (otherwise known as the daughter) and Helium is the α-particle. Always remember that charge and mass have to be conserved. Here isotope Y has lost 2 protons (2 positive charges) and helium has gained them. Isotope X transforms into isotope Y with 4 less nucleons (A − 4) and 2 less protons (Z − 2).
Alpha radiation is frequently accompanied by gamma radiation because of the difference in energies of the particles: photon emission by gamma ray is how energy is conserved.
In other words, when an excited nucleus X* with a star to mean that it's excited, returns to its ground state X, it emits one (or many) gamma-rays by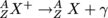 .
.
Where does the electron come from? This is the reaction that left physicists gasping for air in the 1920's. What the heck are electrons doing emerging from decaying nuclei?
Below is the reaction itself: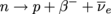 In plain English, a neutron within a nucleus flips into a proton, releasing a β-particle (electron) and an anti-neutrino. Ignore the type of neutrino for now—it's the mutation of neutron to proton we're interested in.
In plain English, a neutron within a nucleus flips into a proton, releasing a β-particle (electron) and an anti-neutrino. Ignore the type of neutrino for now—it's the mutation of neutron to proton we're interested in.
Charge has to be conserved, and mass-energy equivalence has to be conserved too. For that to happen, the net charge has to remain neutral: hence the emission of a negative electron with the creation of a positive proton. We may also assume that the neutrino carries the extra energy given off from the conversion.
Let's not forget the electron is also a lepton, so it has a lepton charge of +1. Every lepton has an anti-lepton, the lepton anti-neutrino created in this case, not that we're interested in types of neutrinos just yet. Anti-leptons have a lepton charge of -1. It's different than electromagnetic charge, though. Electromagnetically neutrinos are neutral. It's okay to be confused right now. Rest assured: the electric charge is conserved, as well as the lepton charge. All is right with the world, er beta-decay.
What about the baryons, the neutron and proton? One baryon's on one side and another's on the other side. The numbers are equal. We're good.
Electromagnetic charge, lepton charge, baryon number, energy, and momentum are all conserved.
A neutron doesn't turn into a proton without a reason, and that reason is the ratio of protons to neutrons in a nucleus. A while back we looked at a graph of binding energy per nucleon as a function of atomic number and found that Iron-56 is the happiest nucleus because it's the most stable, with the most tightly bound nucleons. Nuclei which find themselves with too many neutrons for stability may decide to turn one into a proton through beta-decay.
To write β-decay in terms of atomic configurations, we say that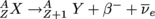 , where X is the parent nucleus and Y is the daughter.
, where X is the parent nucleus and Y is the daughter.  . Here a proton flips into a neutron, releasing an anti-electron, also known as a positron, with the opposite charge of an electron, and an electron-neutrino. Charge, etc. are all still conserved. Using A's and Z's, we can also write that
. Here a proton flips into a neutron, releasing an anti-electron, also known as a positron, with the opposite charge of an electron, and an electron-neutrino. Charge, etc. are all still conserved. Using A's and Z's, we can also write that 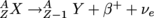 .
.
The types of nuclei that emit positrons have too few neutrons for stability. And yes, the β- radiation happens when nuclei have too many neutrons for stability. Any ideas about what's happening within this easy flipping of neutrons into protons, and vice-versa?
One of the up quarks of the proton flips into a down quarkto make a neutron, and one of the down quarks of the neutron flips into an up quark to make a proton. It begs the question of how that happens, but we're not going there right now. That's for graduate school.
An example of beta-decay with carbon-14 can be seen below: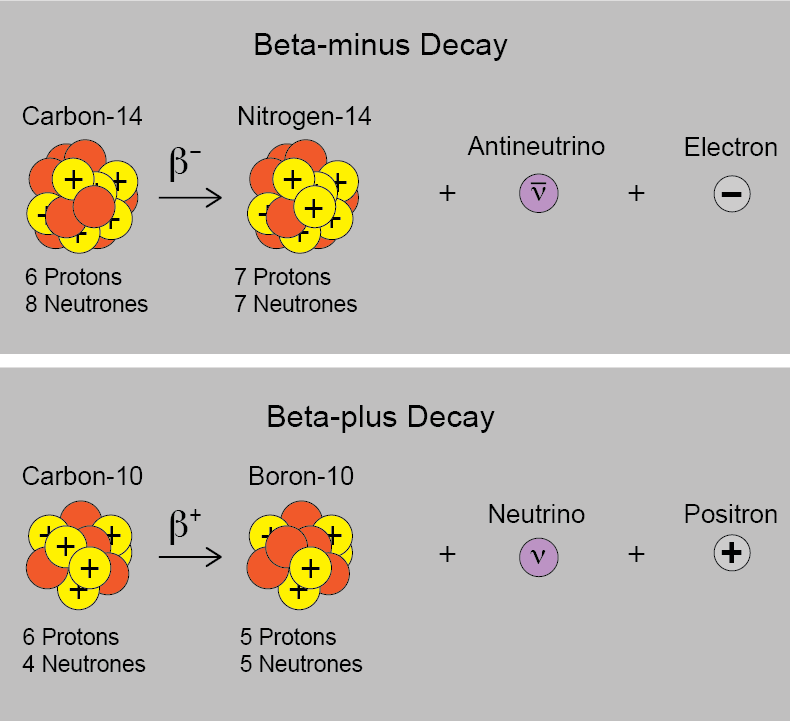
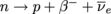 . Rearranging a bit, and keeping in mind our laws of the conservation of baryon, lepton, and electric charge, we may express electron capture as
. Rearranging a bit, and keeping in mind our laws of the conservation of baryon, lepton, and electric charge, we may express electron capture as 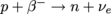 .
.
Putting the electron (as β-) on the opposite side of the equation switched the type of neutrino to conserve lepton charge, not that we particular care about neutrino types right now. All we want is a lepton on either side or a lepton and anti-lepton on the same side, so it all works out.
It takes more energy for a nucleus rich in protons to experience β-decay than for one rich in neutrons, so most nuclei undergo electron capture first, giving it extra energy while ionizing the atom. The electron usually comes from the n = 1 or n = 2 shells.
After an electron is captured by the nucleus, a "hole" in the orbital shell creates a cascade of outer shell electrons find their way down, emitting an x-ray or some other photon as a result. The ionized atom soon captures another electron to restore its neutral charge as well.
Why don't scientist just call β-particles electrons, e? That's a very, very good question. If nothing else, using β alerts us to the fact we're talking about radiation from a nucleus, as opposed to an electron from somewhere else.
Both reactors above were condemned by engineers as flawed, so there's that. While fission reactors have their limitations, some designs are better than others and we don't have to fear them all. The benefits to this modern energy source, however, are not as frequently discussed as the bad endings. Let us do so now, after an introduction to what fission is.
Fission occurs when a heavy nucleus splits into two lighter daughter nuclei, releasing energy due to a significant mass defect, as determined by E = mc2.
Naturally-occurring fission is extremely rare. Most of the time, a heavy nucleus absorbs a high-energy particle or photon before it splits. While a neutron may have high energy, they are difficult to capture because…neutral. They pass right through all sorts of materials without interaction.
Yet a neutron is what causes fission, sourced by a decaying heavy nucleus which includes neutrons as a byproduct instead of the usual α, β, and γ.
For fission to become a likely event, there has to be so many neutrons around that the Uranium or Plutonium, or whatever the fissile material chosen, will absorb a neutron, setting off a chain reaction such that once one fission happens, it fuels more fission because of the added neutrons given off in the fission reaction.
A possible fission reaction involves a Uranium-235 atom absorbing a neutron as shown below. The daughter nuclei X and Y vary, as does the number of released neutrons, x.
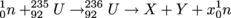
Uranium-236 lasts only about 10-12 seconds, 1 ps, as the nucleus is highly excited. As it oscillates, the nucleus deforms into a dumbbell shape with two positively charged ends. The Coulomb force takes over and tears apart the two ends into lighter nuclei called fission fragments, releasing other neutrons.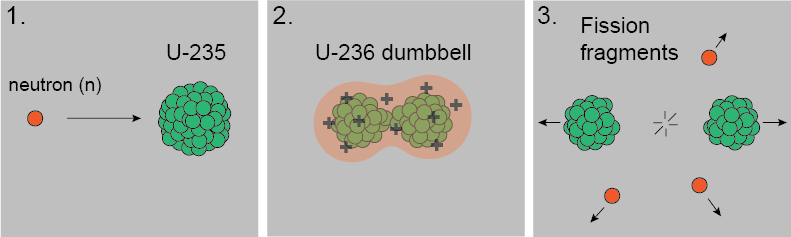 Generally, the two fragments or daughters from U-236 aren't the same size. Here's a diagram.
Generally, the two fragments or daughters from U-236 aren't the same size. Here's a diagram.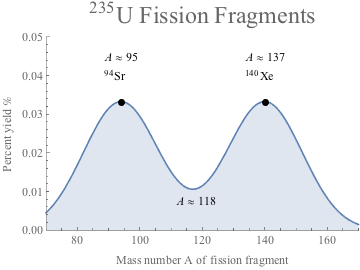 Reading the charg above, Uranium-235 plus a neutron may split into Strontium-95 and Xenon-140, as one of many possibilities. We can see from the percent yield on the y-axis that this type of fission is more likely to give products each having mass numbers of ~95 and ~137. These two fragments are lighter and require less binding energy than the original Uranium nucleus, which means the excess binding energy of U-235 is released as gamma radiation. In addition, fission products are extremely unstable with extra neutrons themselves, which means they undergo β-decay or α-decay and emit even more radiation in a chain reaction, like this, shown below with β-decay:
Reading the charg above, Uranium-235 plus a neutron may split into Strontium-95 and Xenon-140, as one of many possibilities. We can see from the percent yield on the y-axis that this type of fission is more likely to give products each having mass numbers of ~95 and ~137. These two fragments are lighter and require less binding energy than the original Uranium nucleus, which means the excess binding energy of U-235 is released as gamma radiation. In addition, fission products are extremely unstable with extra neutrons themselves, which means they undergo β-decay or α-decay and emit even more radiation in a chain reaction, like this, shown below with β-decay: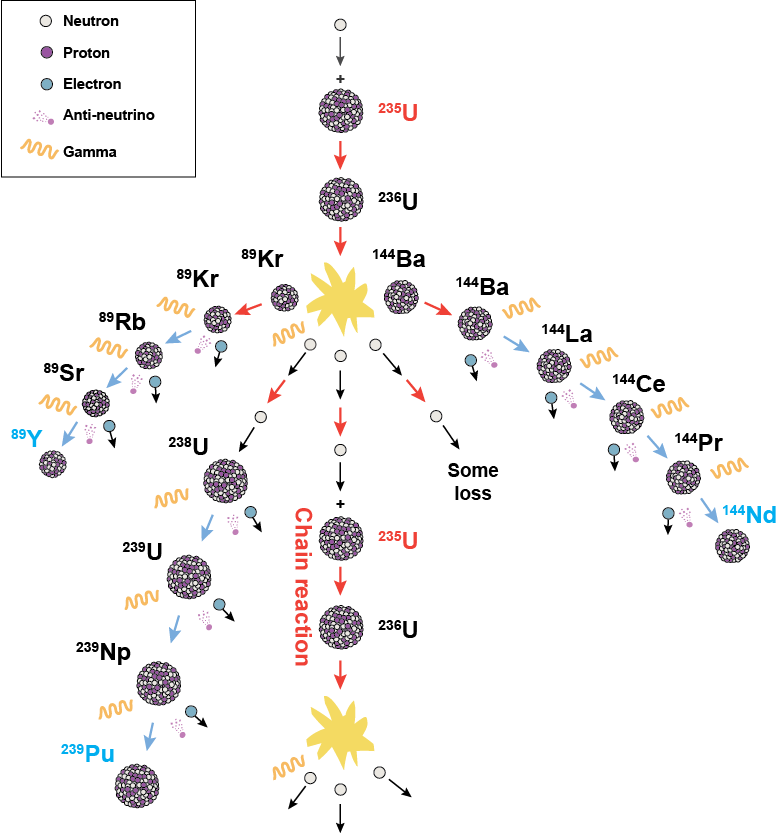 Look at all that energy to be harnessed from so many particles: it's clean, it's cheap, and as long as the fission reactor is designed and maintained properly, there's nearly negligible risk of radiation to the public. France's power is 80% nuclear for quite a few decades now, for example, and they're accident-free.
Look at all that energy to be harnessed from so many particles: it's clean, it's cheap, and as long as the fission reactor is designed and maintained properly, there's nearly negligible risk of radiation to the public. France's power is 80% nuclear for quite a few decades now, for example, and they're accident-free.
Radioactive waste is a problem, though, because once the fissile material begins to be spent, the concentration of fissile material left isn't sufficient to sustain chain reactions, at about 90% strong. Therefore, highly radioactive waste needs a place to go where it can't hurt anyone. Any takers? Us neither.
A second type of fission reactor gaining attention nowadays uses liquid uranium as a molten uranium salt, and spends up to 90% of its fuel before losing the ability to sustain a chain reaction, as opposed to the 10% from the typical fuel rod reactors. Less waste, more efficiency. Plus, this type of reactor isn't as prone to meltdowns, what happens when the fission in a fuel-rod reactor escalates from too much fuel. This molten salt reactor sounds like a win-win-win-winner!
Check it out. We hope they become wildly popular.
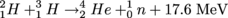
Note that there are other versions with other combinations of Hydrogen isotopes to form Helium, Above, the two Hydrogen atoms are heavy versions, with one and two neutrons, respectively, called deuterium and tritium. They're rare in comparison to the neutron-free version of Hydrogen, but not horrendously difficult to come by, so why aren't we using fusion reactors?
Fusion reactors haven't become practical yet, because they require more input energy than they give out, and 17.6 MeV is a high output per incident so that's saying something. Not ideal for a power source, we'd say.
Those two positive Hydrogen nuclei repel each other strongly, according to Coulomb's Law, and particles are nothing if not law-abiding. To overcome the Coulomb force, the tritium and deuterium must be supplied with so much energy that they travel so quickly that even Coulomb's Law can't force them apart before collision. Yes, we're talking relativistic speeds.
While the Sun and all other stars have the sheer numbers of high energy Hydrogen atoms to make fusion possible, Earth just doesn't. Scientists have been pursuing the elusive "cold fusion" for years, and by "cold" they mean less energy put in than they get out.
Fusion offers the advantage of no radioactive waste over fission reactors, and more energy to harness per event. Maybe we'll see the development of a fusion reactor in the next few decades. Stranger things have happened…. Invisibility cloaks, we're looking at you.
In our special relativity module, we derived an energy-momentum relation for a relativistic particle given by E2 = m2c4 + p2c2, where E, m, and p are the particle's energy, mass and momentum respectively.
From a math standpoint, what are the two possible values of energy for this relativistic equation? Well, that's fairly easy. Just take the square root, and we end up with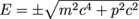 . That plus-or-minus sign indicates interesting results within the world of particle physics.
. That plus-or-minus sign indicates interesting results within the world of particle physics.
We'd be inclined to dismiss the negative energy, ourselves, and go with the positive value. In 1928, while deriving some fancy quantum mechanics, the physicist Paul Dirac encountered this equation. He didn't dismiss anything. In fact, after many hours of headaches and more derivations, he concluded that negative energy states implied the existence of a positron: an electron of mass me but carrying a positive +e charge.
The positron was experimentally discovered in 1932, but as it turns out, every particle out there has an anti-particle of the same mass but opposite in electric and magnetic properties. This is anti-matter.
When matter collides with anti-matter, each annihilates into a burst of energy, equivalent to the energy converted from their respective masses from E = mc2. What would modern physics be without Einstein? Anyways, this energy is released as photons. If an electron β- collides with a positron β+, for instance, then two gamma-rays are released, .
.
The β-particles have opposite charges from each other but each has a mass of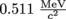 . Multiply our masses by c2 and we're left with energies of E = mc2 =
. Multiply our masses by c2 and we're left with energies of E = mc2 = 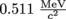 for each of the two photons, on behalf of each of the two particles. The two photons are also necessary to conserve momentum: they can't join into one photon with twice the energy.
for each of the two photons, on behalf of each of the two particles. The two photons are also necessary to conserve momentum: they can't join into one photon with twice the energy.
Graphically, this is what happens: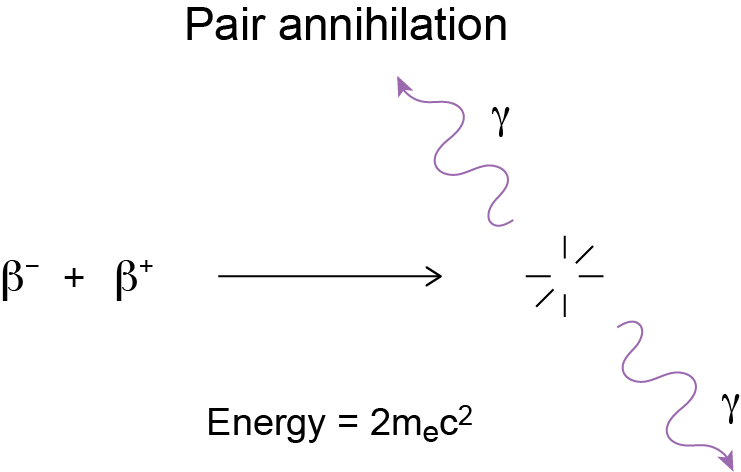 That's matter annihilation in a nutshell. It can happen with any other pair as well: protons and anti-protons, muons and anti-muons, neutrinos and anti-neutrinos, etc.
That's matter annihilation in a nutshell. It can happen with any other pair as well: protons and anti-protons, muons and anti-muons, neutrinos and anti-neutrinos, etc.
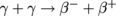 .
.
As long as the two photons have enough energy between them, or the collision of a photon with a nucleus has enough energy, then pair production is possible. All that's required is enough energy to create the two particles with at least their rest mass,. For an electron and a positron, that's apiece. If it's something heavier, even more energy is required, or if lighter such as for neutrinos, not much energy is necessary.
apiece. If it's something heavier, even more energy is required, or if lighter such as for neutrinos, not much energy is necessary.
Pair production occur with any of the elementary particles, which include all other leptons. It's also possible with protons and other composite particles, but there are additional complications to consider.
In any pair production or annihilation the energy, momentum, electric charge, and lepton number are always conserved just as for α- and β-decay.
Elementary versus Composite Particles
Doesn't it seem strange that the proton and neutron each had masses of mp = 1.007825 u and mn = 1.008665 u respectively, while the electron only weighs me = 5.49 × 10-4 u?It certainly seemed bizarre to us. So bizarre that we had to send in one of our Shmoop particle detectives to investigate this large mass difference.
Something with a larger mass might be heavier. We say might because it also depends on density. Protons and neutrons, as it turns out, are definitely larger than an electron. In fact, an electron doesn't even have a definite size, since they're described by a wave function and quantum properties just as much as by particle ones.
As far as we know and, more importantly, as far as scientists know, we can't break an electron into any smaller pieces. The electron is thus an elementary particle. It's also a lepton, subject to the weak nuclear force, but not the strong one. All leptons happen to be elementary particles. The leptons are: electrons, muons, taus, positrons,which are the opposite of electrons, and neutrinos.
Nucleons, on the other hand, aren't elementary particles, they are composite particles. It's a twist, we know, and it may come as a surprise, but neutrons and protons are made up of other particles: quarks.
Like electrons, quarks are elementary particles too, and there are several kinds of quarks. Quarks are not leptons, though, they are hadrons, which means they are subject to the strong nuclear force, but not the weak one. A baryon is a particle composed of three quarks. The two most famous baryons are—we knew it—protons and neutrons.
Let's back up for a moment and talk about forces. Some leptons and hadrons obey the electromagnetic force, but not all of them. To feel the electromagnetic force, a particle must have a charge. Neutrinos (a lepton) are neutral; neutrons (a hadron) have no charge either; but the electromagnetic force does not bother with neutrinos and neutrons. However, the weak and strong nuclear forces certainly bother with them. That tells us that the strong and weak forces are independent of the electromagnetic force. Now, let's go back to talking about quarks.
We just learned that both neutrons and protons are baryons with three quarks, but protons, unlike the neutral neutrons, have a positive charge. How can this be? How can some baryons be charged while others aren't? That's because the quarks are different for protons and neutrons.
Quarks can take on six types of flavors, and yes, they're actually called flavors. We don't have to worry about most of them. The only flavors we care about are up quarks and quarks. Up quarks have a charge of
 . Down quarks have a charge of
. Down quarks have a charge of 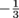 . Physicist discovered this through experimentation with particle accelerators in the 90's.
. Physicist discovered this through experimentation with particle accelerators in the 90's.
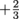 in all the up quarks, and a
in all the up quarks, and a  in the down ones, and lo and behold, protons have a charge of + 1 and neutrons have no charge.
in the down ones, and lo and behold, protons have a charge of + 1 and neutrons have no charge. Radiation from a Particular Point of View
At its core, most of particle physics is radiation because it's about sorts of particles roaming free, free to interact with all sorts of materials including atoms and nuclei. Not that it's limited to free particles, in reality, because particle physics exists wherever particles interact. Let's take a look at alpha, beta, and gamma radiation again while considering the particles at play.Alpha Decay
In this decay, a radioactive isotope emits an α-particle, which is a Helium nucleus with 2 neutrons, 2 protons, and no electrons has a positive +2 charge. This α-particle can be written asWhen an unstable radioactive isotope undergoes alpha decay, it loses 2 neutrons and protons and decays into something else. Let's take a look at the following reaction:
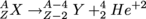
where X is the unstable radioactive parent isotope, Y is the product (otherwise known as the daughter) and Helium is the α-particle. Always remember that charge and mass have to be conserved. Here isotope Y has lost 2 protons (2 positive charges) and helium has gained them. Isotope X transforms into isotope Y with 4 less nucleons (A − 4) and 2 less protons (Z − 2).
Alpha radiation is frequently accompanied by gamma radiation because of the difference in energies of the particles: photon emission by gamma ray is how energy is conserved.
Gamma Decay
Alpha and beta decay most often leave a nucleus in an excited state. To get to the ground state, a nucleus must emit γ-rays, which are on the order of MeV, which corresponds to the frequency of gamma rays and sometimes x-rays as determined by E = hf.In other words, when an excited nucleus X* with a star to mean that it's excited, returns to its ground state X, it emits one (or many) gamma-rays by
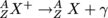
Beta-Decay
We learned earlier that beta-decay involves the emission of electrons from a nucleus, but we didn't study the process behind it. The electron emitted isn't coming from the electron orbitals. No ions are involved with beta-decay. It's all within the nucleus, and no sensible nucleus keeps an electron inside itself.Where does the electron come from? This is the reaction that left physicists gasping for air in the 1920's. What the heck are electrons doing emerging from decaying nuclei?
Below is the reaction itself:
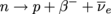
Charge has to be conserved, and mass-energy equivalence has to be conserved too. For that to happen, the net charge has to remain neutral: hence the emission of a negative electron with the creation of a positive proton. We may also assume that the neutrino carries the extra energy given off from the conversion.
Let's not forget the electron is also a lepton, so it has a lepton charge of +1. Every lepton has an anti-lepton, the lepton anti-neutrino created in this case, not that we're interested in types of neutrinos just yet. Anti-leptons have a lepton charge of -1. It's different than electromagnetic charge, though. Electromagnetically neutrinos are neutral. It's okay to be confused right now. Rest assured: the electric charge is conserved, as well as the lepton charge. All is right with the world, er beta-decay.
What about the baryons, the neutron and proton? One baryon's on one side and another's on the other side. The numbers are equal. We're good.
Electromagnetic charge, lepton charge, baryon number, energy, and momentum are all conserved.
A neutron doesn't turn into a proton without a reason, and that reason is the ratio of protons to neutrons in a nucleus. A while back we looked at a graph of binding energy per nucleon as a function of atomic number and found that Iron-56 is the happiest nucleus because it's the most stable, with the most tightly bound nucleons. Nuclei which find themselves with too many neutrons for stability may decide to turn one into a proton through beta-decay.
To write β-decay in terms of atomic configurations, we say that
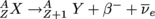 , where X is the parent nucleus and Y is the daughter.
, where X is the parent nucleus and Y is the daughter. Beta-plus Decay
Instead of emitting an electron, some types of β-radiation emit the opposite by the opposite process: with the mutation of a proton into a neutron or . Here a proton flips into a neutron, releasing an anti-electron, also known as a positron, with the opposite charge of an electron, and an electron-neutrino. Charge, etc. are all still conserved. Using A's and Z's, we can also write that
. Here a proton flips into a neutron, releasing an anti-electron, also known as a positron, with the opposite charge of an electron, and an electron-neutrino. Charge, etc. are all still conserved. Using A's and Z's, we can also write that 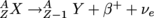 .
.The types of nuclei that emit positrons have too few neutrons for stability. And yes, the β- radiation happens when nuclei have too many neutrons for stability. Any ideas about what's happening within this easy flipping of neutrons into protons, and vice-versa?
One of the up quarks of the proton flips into a down quarkto make a neutron, and one of the down quarks of the neutron flips into an up quark to make a proton. It begs the question of how that happens, but we're not going there right now. That's for graduate school.
An example of beta-decay with carbon-14 can be seen below:

Electron Capture
Electron capture is, in a way, a type of inverse β-decay. As its name of "electron capture" implies, an electron residing near the center of the atom gets gobbled up by the nucleus. This only happens for nuclei that have an excess of protons in comparison to neutrons, but this process is distinct from β+ emission. To refresh our memories of β-decay, we know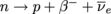 . Rearranging a bit, and keeping in mind our laws of the conservation of baryon, lepton, and electric charge, we may express electron capture as
. Rearranging a bit, and keeping in mind our laws of the conservation of baryon, lepton, and electric charge, we may express electron capture as 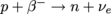 .
.Putting the electron (as β-) on the opposite side of the equation switched the type of neutrino to conserve lepton charge, not that we particular care about neutrino types right now. All we want is a lepton on either side or a lepton and anti-lepton on the same side, so it all works out.
It takes more energy for a nucleus rich in protons to experience β-decay than for one rich in neutrons, so most nuclei undergo electron capture first, giving it extra energy while ionizing the atom. The electron usually comes from the n = 1 or n = 2 shells.
After an electron is captured by the nucleus, a "hole" in the orbital shell creates a cascade of outer shell electrons find their way down, emitting an x-ray or some other photon as a result. The ionized atom soon captures another electron to restore its neutral charge as well.
Why don't scientist just call β-particles electrons, e? That's a very, very good question. If nothing else, using β alerts us to the fact we're talking about radiation from a nucleus, as opposed to an electron from somewhere else.
Fission and Fusion
No discussion of nuclear or particle physics could be complete without talking about two other sources of radiation: nuclear fission and nuclear fusion. We saved the best for last.Nuclear Fission
Fukushima and Chernobyl bring dread into our hearts because we fear that the same could happen to any nuclear reactor. Certainly fission creates destructive bombs, as in the two dropped on Japan to close out WWII.Both reactors above were condemned by engineers as flawed, so there's that. While fission reactors have their limitations, some designs are better than others and we don't have to fear them all. The benefits to this modern energy source, however, are not as frequently discussed as the bad endings. Let us do so now, after an introduction to what fission is.
Fission occurs when a heavy nucleus splits into two lighter daughter nuclei, releasing energy due to a significant mass defect, as determined by E = mc2.
Naturally-occurring fission is extremely rare. Most of the time, a heavy nucleus absorbs a high-energy particle or photon before it splits. While a neutron may have high energy, they are difficult to capture because…neutral. They pass right through all sorts of materials without interaction.
Yet a neutron is what causes fission, sourced by a decaying heavy nucleus which includes neutrons as a byproduct instead of the usual α, β, and γ.
For fission to become a likely event, there has to be so many neutrons around that the Uranium or Plutonium, or whatever the fissile material chosen, will absorb a neutron, setting off a chain reaction such that once one fission happens, it fuels more fission because of the added neutrons given off in the fission reaction.
A possible fission reaction involves a Uranium-235 atom absorbing a neutron as shown below. The daughter nuclei X and Y vary, as does the number of released neutrons, x.
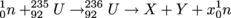
Uranium-236 lasts only about 10-12 seconds, 1 ps, as the nucleus is highly excited. As it oscillates, the nucleus deforms into a dumbbell shape with two positively charged ends. The Coulomb force takes over and tears apart the two ends into lighter nuclei called fission fragments, releasing other neutrons.



Radioactive waste is a problem, though, because once the fissile material begins to be spent, the concentration of fissile material left isn't sufficient to sustain chain reactions, at about 90% strong. Therefore, highly radioactive waste needs a place to go where it can't hurt anyone. Any takers? Us neither.
A second type of fission reactor gaining attention nowadays uses liquid uranium as a molten uranium salt, and spends up to 90% of its fuel before losing the ability to sustain a chain reaction, as opposed to the 10% from the typical fuel rod reactors. Less waste, more efficiency. Plus, this type of reactor isn't as prone to meltdowns, what happens when the fission in a fuel-rod reactor escalates from too much fuel. This molten salt reactor sounds like a win-win-win-winner!
Check it out. We hope they become wildly popular.
Nuclear Fusion
Fusion is the opposite of fission: instead of breaking a nucleus into pieces, it's the combining of two nuclei into one. Two Hydrogens may form Helium, like so, with the output energy coming from the energy released as mass is converted to energy according to E = mc2.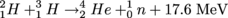
Note that there are other versions with other combinations of Hydrogen isotopes to form Helium, Above, the two Hydrogen atoms are heavy versions, with one and two neutrons, respectively, called deuterium and tritium. They're rare in comparison to the neutron-free version of Hydrogen, but not horrendously difficult to come by, so why aren't we using fusion reactors?
Fusion reactors haven't become practical yet, because they require more input energy than they give out, and 17.6 MeV is a high output per incident so that's saying something. Not ideal for a power source, we'd say.
Those two positive Hydrogen nuclei repel each other strongly, according to Coulomb's Law, and particles are nothing if not law-abiding. To overcome the Coulomb force, the tritium and deuterium must be supplied with so much energy that they travel so quickly that even Coulomb's Law can't force them apart before collision. Yes, we're talking relativistic speeds.
While the Sun and all other stars have the sheer numbers of high energy Hydrogen atoms to make fusion possible, Earth just doesn't. Scientists have been pursuing the elusive "cold fusion" for years, and by "cold" they mean less energy put in than they get out.
Fusion offers the advantage of no radioactive waste over fission reactors, and more energy to harness per event. Maybe we'll see the development of a fusion reactor in the next few decades. Stranger things have happened…. Invisibility cloaks, we're looking at you.
Matter and Anti-Matter
Now that we've heard of anti-electrons, also known as positrons, we recall hearing of anti-matter in science-fiction films. It's supposed to be this cool mysterious substance, perhaps capable of destroying the entire world or even the entire universe depending on the storyline. Sorry, Hollywood, that's not exactly the case.In our special relativity module, we derived an energy-momentum relation for a relativistic particle given by E2 = m2c4 + p2c2, where E, m, and p are the particle's energy, mass and momentum respectively.
From a math standpoint, what are the two possible values of energy for this relativistic equation? Well, that's fairly easy. Just take the square root, and we end up with
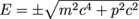 . That plus-or-minus sign indicates interesting results within the world of particle physics.
. That plus-or-minus sign indicates interesting results within the world of particle physics.We'd be inclined to dismiss the negative energy, ourselves, and go with the positive value. In 1928, while deriving some fancy quantum mechanics, the physicist Paul Dirac encountered this equation. He didn't dismiss anything. In fact, after many hours of headaches and more derivations, he concluded that negative energy states implied the existence of a positron: an electron of mass me but carrying a positive +e charge.
The positron was experimentally discovered in 1932, but as it turns out, every particle out there has an anti-particle of the same mass but opposite in electric and magnetic properties. This is anti-matter.
Matter annihilation
Anti-matter threatening to destroy the worldisn't science fiction: there's some truth to it. Dun, dun, dun….When matter collides with anti-matter, each annihilates into a burst of energy, equivalent to the energy converted from their respective masses from E = mc2. What would modern physics be without Einstein? Anyways, this energy is released as photons. If an electron β- collides with a positron β+, for instance, then two gamma-rays are released,
 .
.The β-particles have opposite charges from each other but each has a mass of
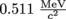 . Multiply our masses by c2 and we're left with energies of E = mc2 =
. Multiply our masses by c2 and we're left with energies of E = mc2 = 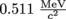 for each of the two photons, on behalf of each of the two particles. The two photons are also necessary to conserve momentum: they can't join into one photon with twice the energy.
for each of the two photons, on behalf of each of the two particles. The two photons are also necessary to conserve momentum: they can't join into one photon with twice the energy.Graphically, this is what happens:

Pair Production
The opposite of matter annihilation occurs as well. Instead of spontaneous combustion, it's spontaneous creation. For example, a positron and an electron may appear from the collision of two photons, as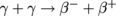 .
.As long as the two photons have enough energy between them, or the collision of a photon with a nucleus has enough energy, then pair production is possible. All that's required is enough energy to create the two particles with at least their rest mass,. For an electron and a positron, that's
 apiece. If it's something heavier, even more energy is required, or if lighter such as for neutrinos, not much energy is necessary.
apiece. If it's something heavier, even more energy is required, or if lighter such as for neutrinos, not much energy is necessary.Pair production occur with any of the elementary particles, which include all other leptons. It's also possible with protons and other composite particles, but there are additional complications to consider.
In any pair production or annihilation the energy, momentum, electric charge, and lepton number are always conserved just as for α- and β-decay.