ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Chemistry Videos 54 videos
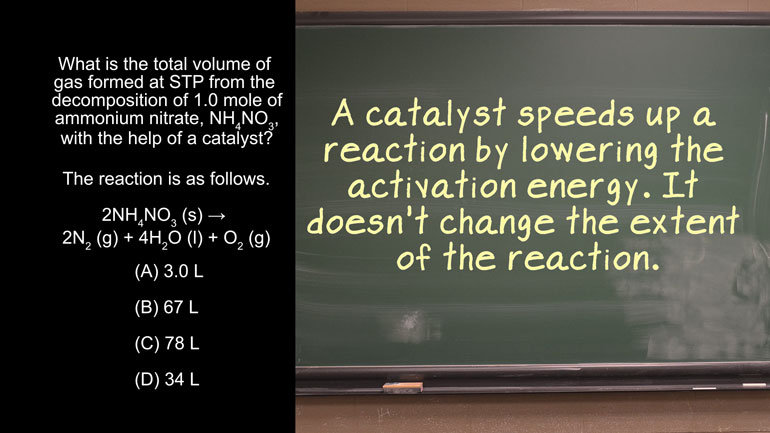
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
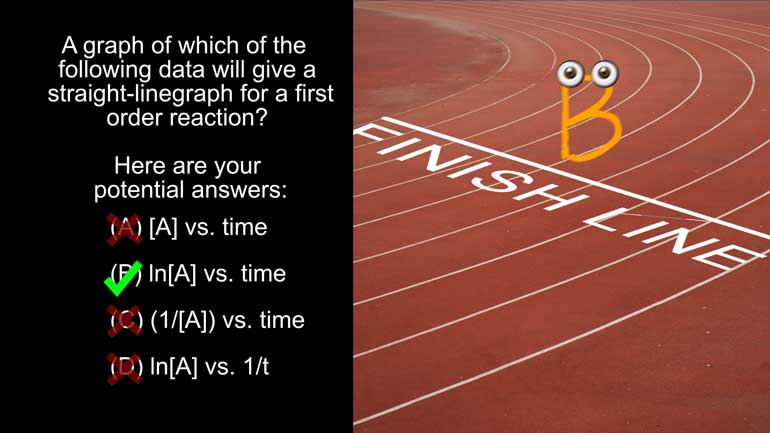
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
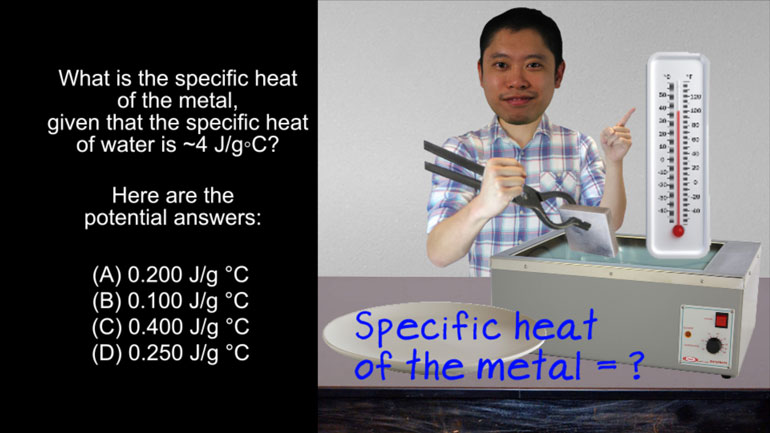
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 1.4 Forming and Breaking Bonds 12 Views
Share It!
Description:
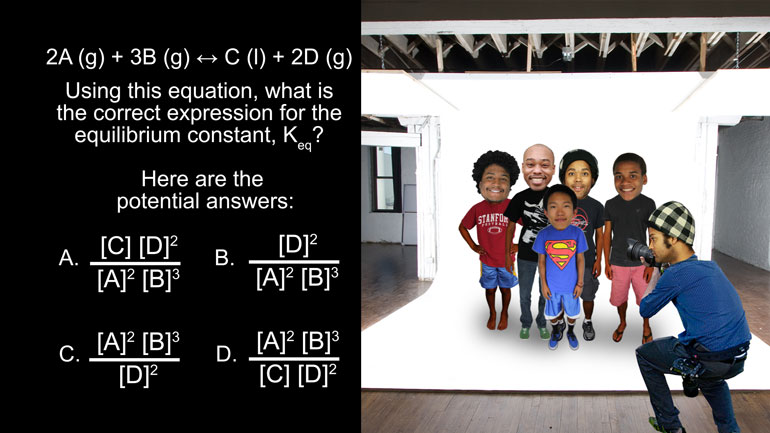
AP Chemistry 1.4 Forming and Breaking Bonds. What is the correct expression for the equilibrium constant, K eq ?
Transcript
- 00:04
Here’s your Shmoop du jour, brought to you by The Jackson 5. They think everything is [Jackson 5 singing]
- 00:08
as easy as ABC, 123… Apparently they never took AP Chemistry. [Jackson 5 in a chemistry class]
- 00:14
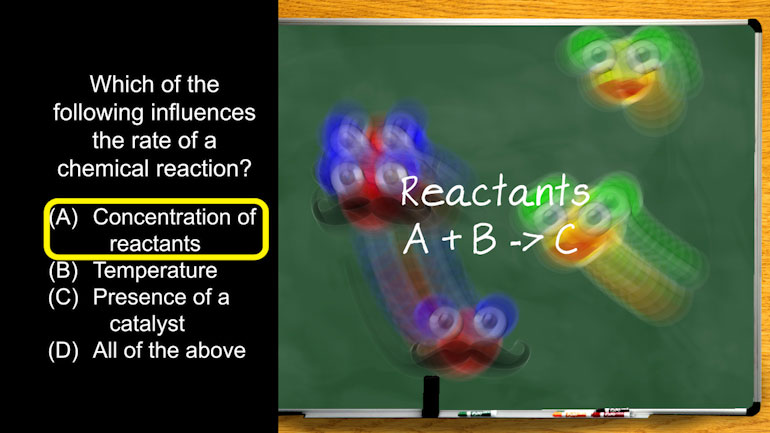
Okay, enough singing and dancing…here’s our question:
- 00:18
Consider the following chemical reaction equation…
- 00:21
Using this equation, what is the correct expression for the equilibrium constant, Keq?
Full Transcript
- 00:28
And here are the potential answers:
- 00:31
Okay, okay, maybe we were a little hard on those Jacksons. This one really is as easy
- 00:37
as ABC… D. Can’t forget D. [Jackson 5 in chemistry class and teacher shows A, B, C, D on board]
- 00:41
Anyway, let’s get to our question. The equilibrium constant for any chemical
- 00:45
reaction will always be the concentrations of the products divided by the concentrations
- 00:48
of the reactants, with each species raised to the power of its stoichiometric
- 00:54
coefficient. Yeah, where's your ABC, 123 now, Jackson Five? [Person taking photo's of Jackson 5]
- 00:57
So for this reaction, the equilibrium constant is C times D squared divided by A squared
- 01:03
times B cubed. But wait, there’s more! Call now, and you’ll [Man selling frying pans]
- 01:07
get a second full set of banana-shaped frying pans for free, just pay $89.95 processing
- 01:12
and handling! …Whoops. Sorry for the confusion, that’s
- 01:14
our other job… tough economic times, right?
- 01:19
But there actually is more… There’s an important trick to this question:
- 01:22
we also need to consider the phase of each reactant and product.
- 01:27
Remember that we only include the reactants and products that are in the gaseous state [Reactants and product put inside a box]
- 01:31
in the equilibrium constant. We exclude species that are liquids or solids.
- 01:37
That means we can’t include species C in the equilibrium constant expression.
- 01:41
So, if we revisit our previous expression for Keq but remove species C, which is a liquid, [Species C removed from formula]
- 01:47
we’re left with D squared divided by A squared times B cubed. And that’s choice B, the
- 01:52
correct answer. Now, who wants some banana-shaped blueberry
- 01:56
pancakes? If we’ve learned one thing today, it’s that this is a deal too good to pass [Man selling banana-shaped blueberry pancakes]
- 02:00
up – don’t wait, call now! …Sorry. We have to meet a quota.
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?