ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Videos 1345 videos
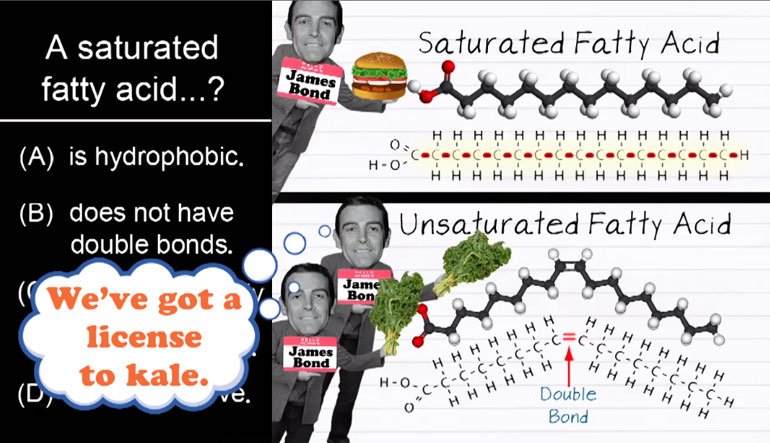
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
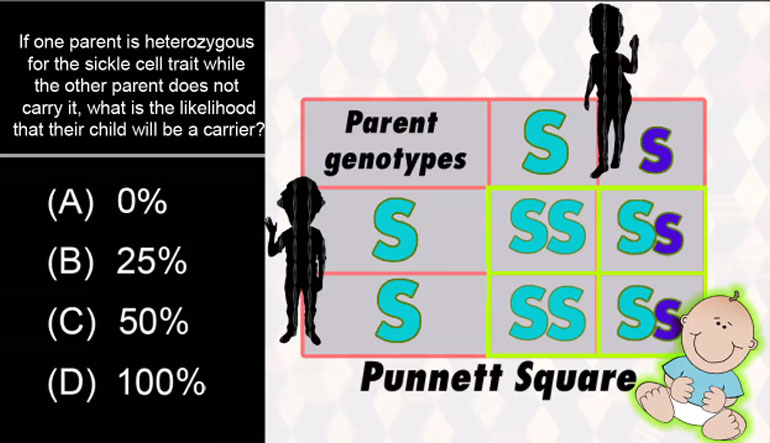
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
AP Biology: Evolution Drives the Diversity and Unity of Life Drill 1, Problem 1. The first cells on planet Earth were likely what?
AP Chemistry 3.4 Structure and Arrangement of Atoms 10 Views
Share It!
Description:
Anyone know what the value of "q" is? We took it into a pawn shop, but the woman just looked at us funny.
Transcript
- 00:04
Here’s your Shmoop du jour, brought to you by Thanksgiving Dinner, bringing atoms together [A thanksgiving dinner on a table and atoms drinking wine]
- 00:09
since 13.82 billion BC!
- 00:12
…Or maybe that was intermolecular forces….
- 00:15
Here’s our question…
- 00:17
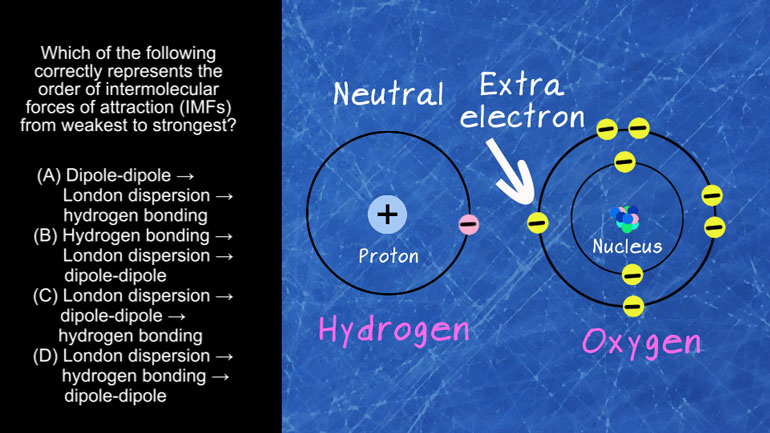
Which of the following correctly represents the order of intermolecular forces of attraction
Full Transcript
- 00:21
(IMFs) from weakest to strongest?
- 00:25
And here are our potential answers…
- 00:32
To answer this question, we first have to understand how each of these intermolecular [Hydrogen bond, dipole dipole and london dispersion having dinner]
- 00:36
forces of attraction work.
- 00:38
That might mean watching a few atom-based romcoms… you know, like Chargeless in Seattle,
- 00:45
or When Harry Met Electron… [Man with atom as a face meets girl with electron as a face]
- 00:47
Or it might just mean watching the rest of this video.
- 00:50
The forces listed in the question are dipole-dipole forces, London Dispersion forces, and hydrogen
- 00:56
bonding.
- 00:57
And since we don't have our copy of Bridget Jones Dipole Diary on us, let's just go through [Man in videostore picking a DVD]
- 01:02
them one by one…
- 01:03
Starting with dipole-dipole forces.
- 01:04
When we’re talking about dipoles, we’re actually talking about two important areas
- 01:08
of small electric charge on a molecule. [Electric charges]
- 01:11
When a bond exists in a molecule between two atoms with very different electronegativities,
- 01:14
the atom with the higher electronegativity hogs a disproportionate amount of electrons. [Atom grabs electronegativity at a table]
- 01:20
Kind of like your cat’s idea of “sharing” furniture space.
- 01:25
Atoms that control more electrons in a bond become more negative in charge.
- 01:29
This leaves the other atom in the bond with a slight positive charge.
- 01:32
Dipole-dipole interactions occur when the slight negative charge of an atom in a dipole [negative charges of a molecule circled]
- 01:36
is attracted to the slight positive charge of an atom in a dipole in a different molecule.
- 01:41
Which means dipole-dipole interactions attract the two molecules together. [Molecules attract to dipole dipole magnet]
- 01:45
Dipole-dipole interactions are reasonably strong intermolecular forces, but the strength
- 01:50
varies depending on the intensity of the dipole. [Dipoles with flexed biceps]
- 01:52
The bigger the difference in electronegativities, the stronger the interaction.
- 01:57
Guess what has a really low electronegativity?
- 01:59
Hydrogen. [Arrow points to hydrogen]
- 02:00
And guess what has a really high electronegativity?
- 02:03
…Well, lots of things, but most commonly Oxygen, Nitrogen, and Fluorine.
- 02:08
And because we're generous 'round these parts, we'll give you three guesses as to what a [Man holding a sign saying 3 guesses]
- 02:12
dipole-dipole interaction in a molecule with a bond between Oxygen, Nitrogen, or Fluorine,
- 02:16
and a Hydrogen is called…
- 02:18
Yup the answer to our super tricky question is hydrogen bonding. [Hydrogen atoms bonding and explosion occurs]
- 02:22
A hydrogen bond is a particularly strong dipole-dipole interaction, because the difference in electronegativity
- 02:28
is so large between Hydrogen and elements like Oxygen or Nitrogen.
- 02:32
This means that in general, hydrogen bonds will be stronger than general dipole-dipole
- 02:36
interactions; however, the two interactions are similar in strength. [Hands touch each other]
- 02:41
Since hydrogen bonds and dipole-dipole interactions are similar in strength, London dispersion [Hydrogen bonds and dipole dipole on a see-saw]
- 02:46
forces must be either stronger than both or weaker than both.
- 02:51
So where do they fall?
- 02:52
Well, London dispersion forces are actually a pretty weak force, because they result from
- 02:57
a property of the electron cloud of a molecule known as its polarizability. [Electron cloud and weather reporter man appears]
- 03:01
That's just a fancy way of saying that the electrons can move around to make little tiny
- 03:07
dipoles….giggity…. [electrons create a dipole in an electron cloud]
- 03:10
This happens if more electrons end up on one side of a molecule for a short period of time.
- 03:14
A good way to compare these forces comes, like many good things, from the kitchen. [Selection of food in a kitchen]
- 03:19
London Dispersion Forces are best approximated by the forces holding together wet spaghetti
- 03:23
noodles, whereas hydrogen bonds are like the forces holding together a single block of [Man holding large blocks of ice]
- 03:27
ice.
- 03:28
You can pull the spaghetti apart with your hands but good luck trying that with ice.
- 03:33
Since London Dispersion Forces are so weak, the answer must be C.
- 03:37
But just because London Dispersion Forces are weak, doesn't mean they can't find love. [London Dispersion Force working out in a gym]
- 03:42
Four Weddings and a London Dispersion Funeral….Coming soon, to a theatre near you.
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?